
Lab Testing
Module Guide - A Deeper Dive!

Introduction
Lab Testing, or LIMS (Laboratory Information Management System) is an optional module within V5 Traceability centered around advanced Quality Control for batch manufacturers.
The software if often used for testing and tracking manufactured batch samples to ensure quality control targets are achieved prior to the finished product packaging phase.
A multitude of Quality Assurance tests can be setup and grouped. Such tests often relate to the physical and chemical analysis of the sample. Samples achieving a ‘PASS’ will authorize the batch to proceed to the packaging phase. Failed batches will trigger a batch rework and resample until a ‘PASS’ grade is achieved. History is automatically digitised.
Succesful batches automatically produce Certificate of Analysis reports, which can accompany the packaged product with the delivery documentation.
Table of Contents
1. Lab Testing Questions Setup
We can set up our Lab Testing questions in the Q&A section of Control Center. We would start in the top left panel and add a new question (1) to be asked during Lab Testing. We would enter our question, ensure that we have ‘Question/Message’ selected from the type column drop-down menu (2). We can also add any group tags we want to apply. In this case we have grouped our lab testing questions by ‘Chemical’ and ‘Physical’ tests. These group tags are purely for labelling purposes so we can keep our questions organized in Control Center.
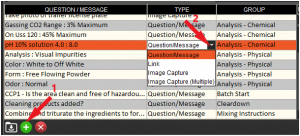
Once this is done, we can go to the top right panel and add event triggers for our Lab Testing questions (1). It is essential here that we select ‘Lab Testing’ from the Event drop-down menu (2). After this we can also select a specific commodity that this question will be asked for, as well as a specific location where we want this question to be asked. We can also leave these as ‘All’ so that the question will be asked for all commodities across all locations.

We can also add more Lab Testing event triggers if we want to specify a number of different commodities or locations where the question will be asked.
For a full breakdown of how all these columns work, please see our Control Center guide here.
Finally, we can use the bottom panel to add our desired answers (1) that will be presented to the operator undertaking the testing. We can then define these (2) and determine whether each answer will ‘Terminate’ the process, ‘Notifies’ management of a recording of this result, or ‘Require (a) Picture’, where the operator will be asked to upload an image if this answer is selected.

2. Lab Testing Made Easy
Ensuring compliant formulations and products, the V5 Traceability Lab Test System forces automatic quarantine of selected finished goods, pending the results from your laboratory testing process.
Standard Functionality
- Ensures all LIMS testing is performed according to product specification.
- Co-ordinate the approval of materials where multiple samples and approvals are required.
- Prevent usage of expired or unapproved materials.
- Create Certificates of Analysis from test results and store results for easy retrieval.
- Testing of in process samples (WIP batches), finished goods or pass through raw materials.
- Customized list of testing parameters, questions and responses.

V5 Traceability allows batches to be sampled multiple times (or once), tracks the results and generates a Certificate of Analysis for all your testing parameters.
3. Laboratory Information Management System (LIMS)
From the main tile menu, a simple user interface presents the user with a choice of produced products and batches which require lab testing.

Once the product is selected for lab testing, the user will be presented with a product image and a selection of questions with Pass / Fail or variable (numeric) input parameters. Questions can be optional or mandatory
Products may be tested once or multiple times (See Certificate of Analysis Detailed Report. The samples average and standard deviation is calculated automatically. Successfully tested products are released from quarantine (hold) and cleared for shipping. COA’s are available to print or email and automatically archived. What is a COA? Find out here.
4. LIMS Sample Hold Labels
Labels can be printed to identify samples currently in Lab Testing mode with the option to place on hold (auto quarantine) and suspended from shipping. Several pre-formatted options exist which satisfy the majority of customer installations. Data matrix symbology is robust and allows multi directional scanning (perfect for industrial applications).
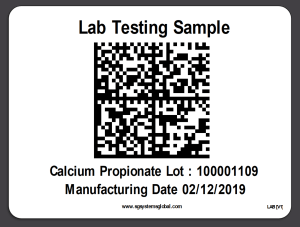
Printing options exist for direct serial, usb and networked printing (wifi or hardwired) and support existing for ZPL (Zebra), IPL (Intermec) plus FTP printing for use with print servers such as Loftware & Bartender.
Lets take a step back – What is LIMS?
A laboratory information management system (LIMS) is a software-based solution with features that support a laboratory’s operations. Key features include — workflows and data tracking support, flexible architecture, and data easy to use exchange interfaces, which fully “support its use in regulated environments”. The software features in LIMS have evolved over the years from simple sample tracking to an enterprise resource planning tool that manages multiple aspects of laboratory informatics.
There is no definition of the term “LIMS” as it is used to encompass a number of different laboratory informatics components. The spread and depth of these components is dependent on the LIMS implementation itself. All LIMS products have a workflow component and some summary data management facilities but beyond that there are significant differences in functionality.

Historically the LIMS, LIS (Laboratory Information System, and process development execution system (PDES) have all performed similar functions. The term “LIMS” has tended to refer to informatics systems targeted for environmental, research, or commercial analysis such as pharmaceutical or petrochemical work. “LIS” has tended to refer to laboratory informatics systems in the forensics and clinical markets, which often required special case management tools. “PDES” has generally applied to a wider scope, including, for example, virtual manufacturing techniques, while not necessarily integrating with laboratory equipment.
Modern LIMS functionality has spread even further beyond its original purpose of sample management. Assay data management, data mining, data analysis, and electronic laboratory notebook (ELN) integration have been added to many LIMS, enabling the realization of translational medicine completely within a single software solution. Additionally, the distinction between LIMS and LIS has blurred, as many LIMS now also fully support comprehensive case-centric clinical data.
For more information on how LIMS can work for your business, contact our sales team here.
